Question 27 Give the symbol of the Period 4 element with smallest atomic radius. The atomic radius of helium is shrinking because it.
So all these elements belong to the 4 t h group and Kr has the smallest atomic radii as it lies across the period.
. Period 4 element with smallest atomic radius. The concentration of more protons in the nucleus creates a higher effective nuclear charge In other words there is a stronger force of attraction pulling the electrons closer to the nucleus resulting in a smaller atomic radius. The element with the smallest atomic radius in period 4 is what.
Helium has the smallest atomic radius at 31 picometers. Helium has the smallest atomic radius. And The atomic radius increases down the group.
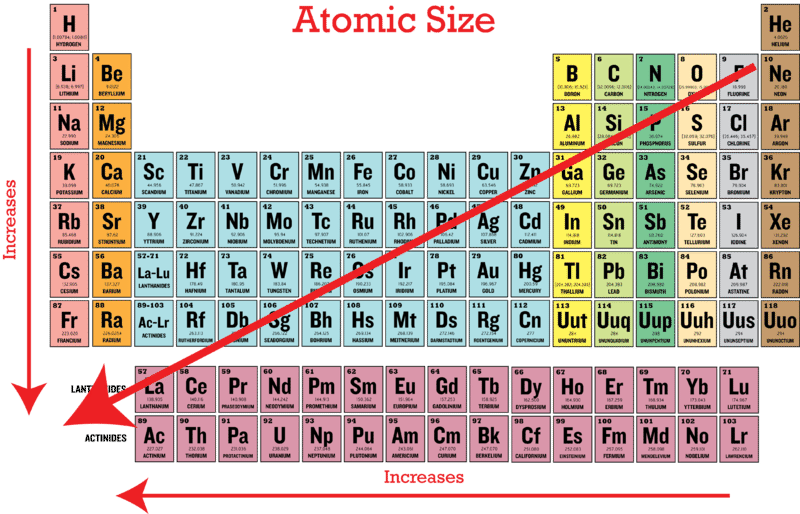
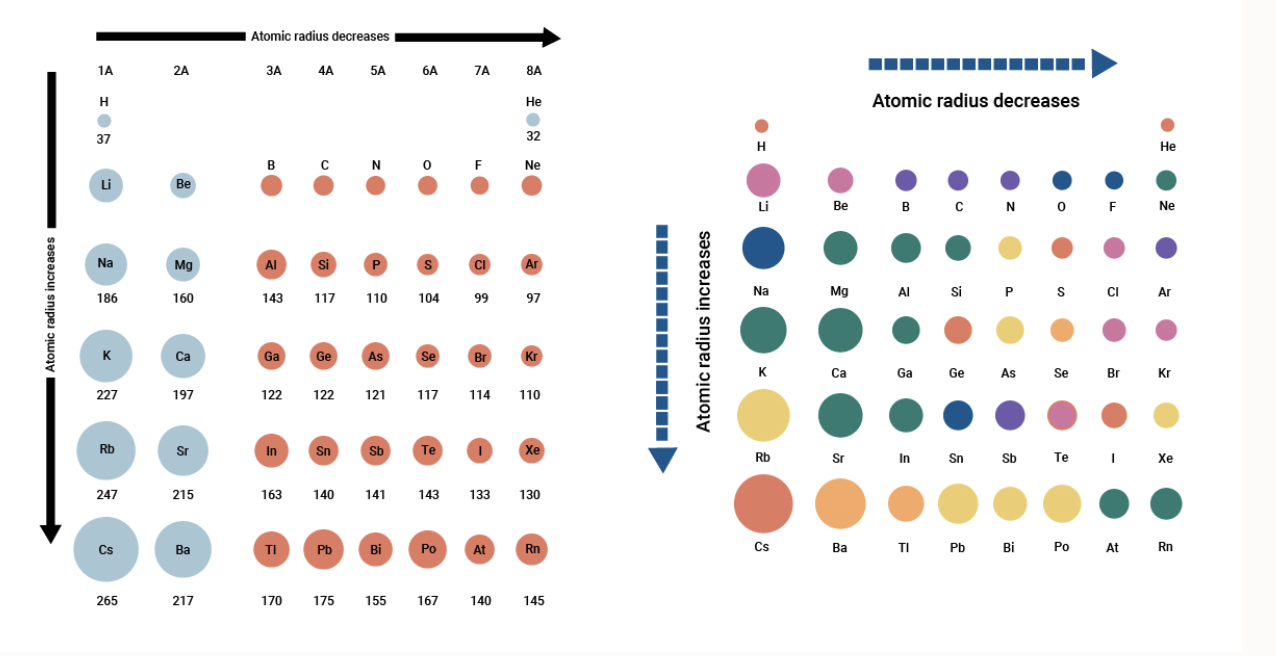
Variation along the period. Which period has the smallest atomic radius. As can be seen in the figures below the atomic radius increases from top to bottom in a group and decreases from left to right across a period.
Thus helium is the smallest element and francium is the largest. Fluorine is the element with smallest atomic radii. 119 rows Atomic radius of Helium He 140 pm.
For example Sodium in period 3 has an atomic radius of 186 picometers and chlorine in the same period has an. Part E period 4 element with the smallest atomic radius Express your answer as a chemical symbol. Which of the rare earth elements is the smallest.
From electron filling of atomic shells we know that the smallest atomic radius will be of the element which has electrons having lowest principal quantum n 1. It is krypton as it the far most right on the periodic table having the smallest radius. Part E period 4 element with the smallest atomic radius Express your answer as a chemical symbol.
O C Be Ne Largest Be Smallest - Ne Explain why you made these choices. Which of the metalloids has the smallest atomic radius. Atomic radius of Lithium Li 182 pm.
Atomic radius decreases as you move across a period from left to right and decreases as you move up a group from bottom to top. Hence the smallest is NNitrogen. Helium is in the top period and the farthest right group which follows the patterns of atomic radius on the periodic table.
Thus the order of size is NaMgBN. Atomic radii vary in a predictable way across the periodic table. Circle the element with the largest atomic radius and put a square around the element with the smallest atomic radius.
Which of the transition metals in the fifth period is the largest. Image showing periodicity of atomic radii Clementi for period 4s 4p and 4d chemical. The element which has the smallest atomic mass is Hydrogen H which has a proton and an electron.
ReasonThis is due to an increase in nuclear charge which tends to pull the. We know that Hydrogen atom has one proton in its nucleus whereas helium atom has two protons. You should consult reference 1 for full details but it is not light reading for most people.
The atomic size decreases towards the right in a period as effective nuclear charge increases. Atomic radii vary in a predictable way across. As such we have only two elements which fill the 1s orbital.
Atomic radius decreases as you go left to right across a period. View the full answer. Pottassium K Calcium Ca Scandium Sc Titanium Ti Vanadium V Chromium Cr Manganese Mn Iron Fe.
O A chemical reaction does not occur for this question Submit Previous Answers Request Answer. - The atomic radius decreases as we move from left to right along the period. The atomic radius decreases as we move from left to right along the period.
Thus helium is the smallest element and francium is the largest. Solve any question of Classification Of Elements And Periodicity In Propertieswith-. The atomic radius of an atom is defined as the average distance.
There is a correlation between the atomic radii as determined from these calculations and the radii of maximum charge density in the outermost shell of the atom. Down the group size increases due to addition of a new shell. Are metal ions larger or smaller than the neutral atoms they came from.
Which element has the smallest atomic radius. 4 rows Which element has the largest atomic radius. This is due to trends in the periodic table and the effective nuclear charge that holds the valence electrons close to the nucleus.
The Atoms of Period 4 from left to right in a Periodic table is given below.

Periodic Trends In Atomic Size Ck 12 Foundation

The Parts Of The Periodic Table

Periodic Trends Review Problems Mass Atomic Radius Electronegativity Etc In 2022 Ionization Energy Electron Configuration Atom
0 Comments